客户采用环氧基磁珠Homo-epoxy偶联亲和配基在《International Journal of Biological Macromolecules》发文
客户采用环氧基磁珠Homo-epoxy偶联亲和配基在《International Journal of Biological Macromolecules》发文
Affinity-assisted covalent self-assembly of PduQ-SpyTag and Nox-SpyCatcher to construct multi-enzyme complexes on the surface of magnetic microsphere modified with chelated Ni2+
Mianxing Luo, Meng Zhang, Changbiao Chi, Guo ChenDepartment of Bioengineering and Biotechnology, Huaqiao University, Jimei Ave. 668, Xiamen 361021, China
Received 8 September 2023, Revised 6 December 2023, Accepted 8 January 2024, Available online 11 January 2024, Version of Record 26 January 2024.
International Journal of Biological Macromolecules
Volume 261, Part 1, March 2024, 129365
https://doi.org/10.1016/j.ijbiomac.2024.129365
Abstract
It is of great significance to study the effect of multi-enzyme aggregation behavior at the interface on the formation of multi-enzyme complexes and their co-catalytic characteristics, which is helpful for us to design and construct immobilized multi-enzyme complex systems for in vitro synthetic biology. Here, a magnetic microsphere with chelated Ni2+, was prepared to explore the self-assembly characteristics of PduQ-SpyTag (P-T) and Nox-SpyCatcher (Nsingle bondC) on its surface, based on the mixed interaction mode consisting the affinity of His-tag/Ni2+ and covalent binding of SpyTag/SpyCatcher. After studying the effect of saturated or unsaturated adsorption of P-T on the covalent binding between P-T and Nsingle bondC at the interface, a possible multienzyme interaction mechanism for the affinity-assisted covalent self-assembly on the Ni2+ chelating surface was proposed. The time evolution of NADH showed that the immobilized P-T/N-C complex formed by this method and the free P-T/N-C complex exhibited similar synergistic catalytic properties, and presented higher catalytic efficiency than the simple mixing of P-T and Nsingle bondC. The optimal catalytic conditions, stability and reusability of the immobilized multi-enzyme complexes prepared in this study were also discussed by comparing them with free enzymes. In this study, we demonstrate a simple and effective strategy for self-assembling SpyTag/SpyCatcher fusion proteins on the surface of magnetic beads, which is inspiring for the construction of more cascade enzyme systems at the interface. It provides a new method for facilitating the rapid construction of immobilized multi-enzyme complexes in vitro from the crude cell lysis.
Keywords
Interfacial self-assemblySpyTag/SpyCatcherMagnetic affinity microsphere
研究界面处多酶聚集行为对多酶复合物形成及其共催化特性的影响具有重要意义,有助于我们设计和构建体外合成生物学的固定化多酶复合物体系。在这里,一个带有螯合Ni的磁性微球2+,基于His-tag/Ni亲和力的混合交互模式,探讨了PduQ-SpyTag(P-T)和Nox-SpyCatcher(N单键C)在其表面的自组装特性2+以及 SpyTag/SpyCatcher 的共价结合。在研究了P-T饱和或不饱和吸附对P-T和N-C单键界面共价结合的影响后,提出了亲和力辅助共价自组装在Ni上可能的多酶相互作用机制2+ 提出了螯合表面。NADH的时间演化表明,该方法形成的固定化P-T/N-C配合物与游离的P-T/N-C配合物表现出相似的协同催化性能,催化效率高于P-T和N-C单键的简单混合。通过与游离酶的比较,讨论了本研究制备的固定化多酶复合物的最佳催化条件、稳定性和可重复使用性。在这项研究中,我们展示了一种在磁珠表面自组装SpyTag/SpyCatcher融合蛋白的简单有效的策略,这对在界面处构建更多的级联酶系统具有启发性。它提供了一种新方法,用于促进 从粗细胞裂解中快速构建体外固定化的多酶复合物。
关键字:界面自组装,SpyTag/SpyCatcher,磁性亲和微球
2.1. Materials
Epoxy-modified magnetic polystyrene microspheres (4.5 μm) was purchased from PuriMag Biotech (Xiamen, China). Iminodiacetic acid (IDA), nickel sulfate hexahydrate (NiSO4·6H2O), sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O), disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) sodium chloride (NaCl), potassium dihydrogen phosphate (KH2PO4), di‑potassium hydrogen phosphate (K2HPO4), sodium carbonate anhydrous (Na2CO3), sodium bicarbonate (NaHCO3), glycerol and Ammonium sulfate ((NH4)2SO4) were purchased from Sinopharm Chemical Reagents Company. LB broth, Ni-IDA column, agarose, peptone, yeast extract, kanamycin, β-nicotinamide adenine dinucleotide trihydrate (NAD) and β-nicotinamide adenine dinucleotide disodium salt (NADH) were purchased from Sangon Biotech (Shanghai, China). 1,3-propanediol (1,3-PD) and dithiothreitol (DTT) were purchased from Aladdin. Restriction enzymes and T4 DNA ligase were purchased from Thermo Fisher Scientific. 5 × SDS-PAGE protein sample loading buffer was purchased from Beyotime Biotechnology (Shanghai, China). All of the primers used in this work were synthesized by Genscript (Nanjing, China).+
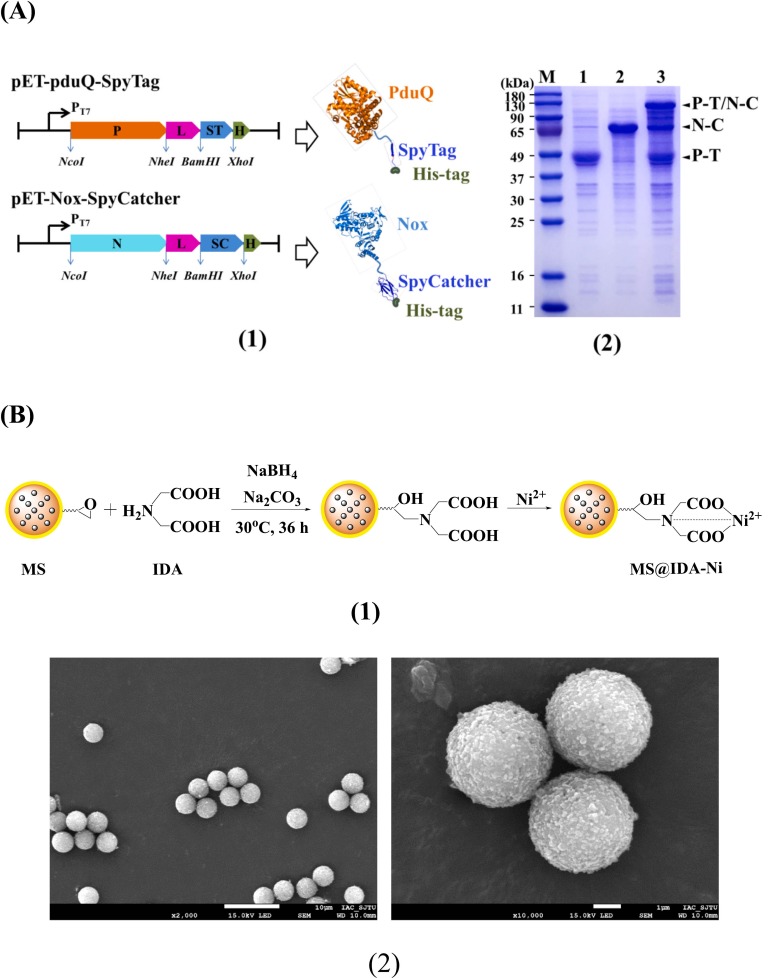
环氧基磁珠请参考: 高密度环氧基磁珠|环氧基磁性微球(1微米)|PuriMag Homo-Epoxy磁珠-生物磁珠专家 (purimagbead.com)
- 上一篇:磁固相萃取系列磁珠PuriMag™ MSPE 小分子前处理利 2024/6/17
- 下一篇:客户在《Plant Physiology and Bioch 2024/5/18
